CLIANTHA RESEARCH LIMITED
Opposite Pushparaj Towers, Near Judges Bungalows, Bodakdev, Ahmedabad-380 054.
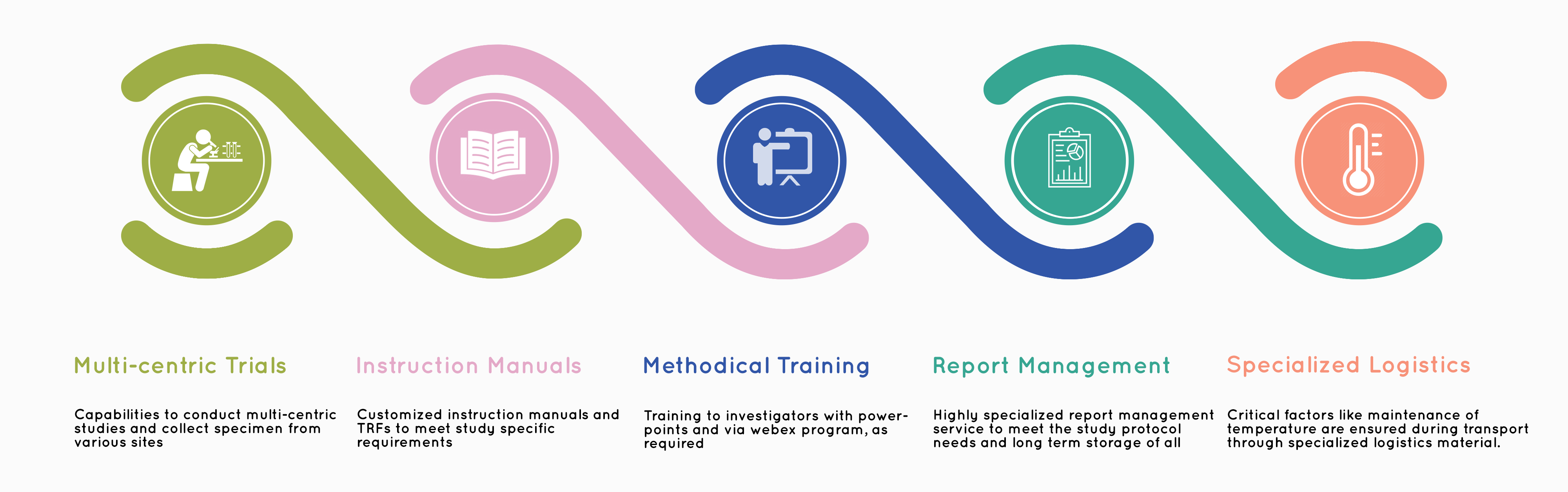
Cliantha Research provides scientific expertise with state-of-the-art technologies reinforced by commitment for quality delivery. Our comprehensive lab services include central lab testing, bioanalytical, Pharmacokinetics (PK) services, Immunogenicity (ADA), Bio marker, Vaccine Immunogenicity testing.
Locations: India & Canada
Cliantha Research has been successfully inspected or audited by various regulatory agencies across the globe. Our impeccable regulatory track record is the result of strict adherence to the applicable standard(s) and guidelines laid by ICH, FDA, EMA, Clinical and Bio-Analytical bodies. Our enshrined values: Science & Integrity are the foundation for our planning and execution assisted with robust knowledge of the regulations.
To know the Regulatory Inspection: click here
Cliantha set up an IVRT laboratory in Toronto in May 2018 to conduct IVRT/IVPT method development and method validation studies for monitoring the release of an API from topical dosage forms, generally semisolid formulations such as creams, ointments, gels etc.
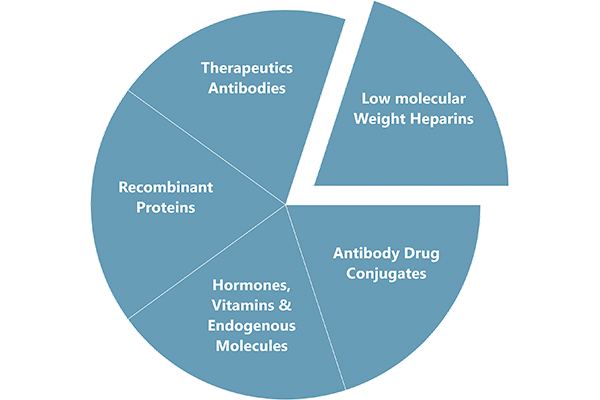
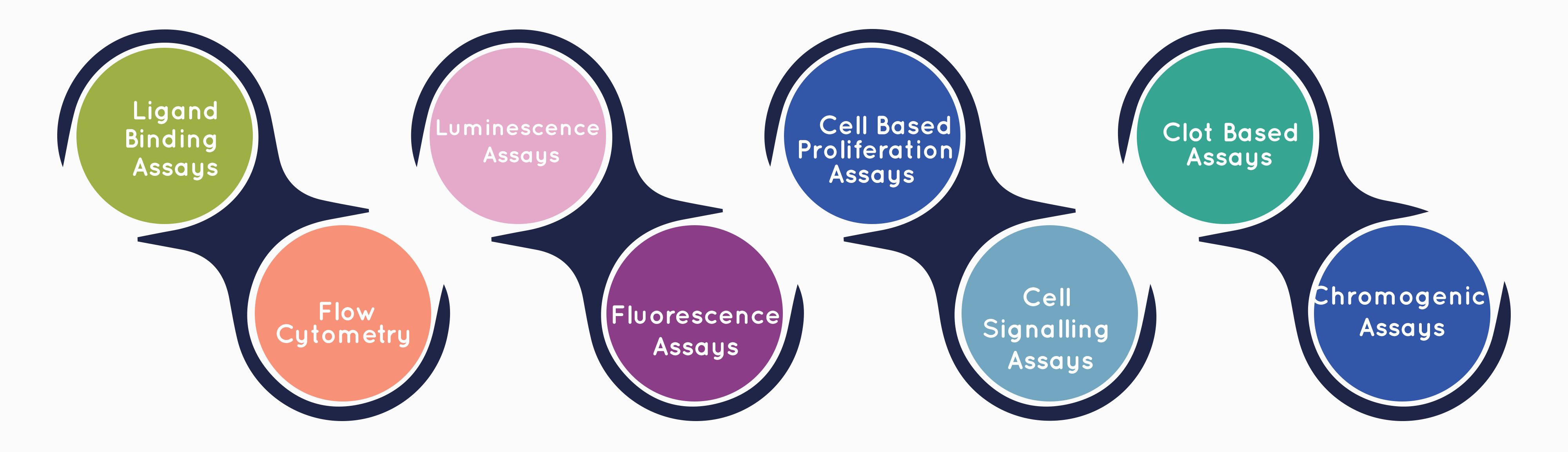
Cliantha’s Large Molecules laboratory has been dedicated to helping clients advance their research programs by providing high quality, accurate results for biosimilar development.






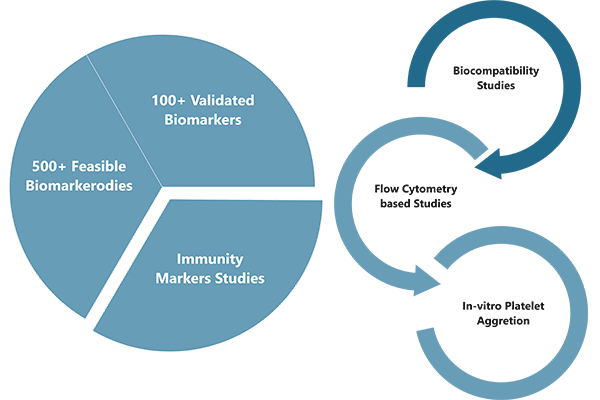
Biomarkers are increasingly used to assess the effects of new drugs and therapeutic biological products in patient populations. Because of the important roles biomarkers can play in evaluating the safety, activity, or effectiveness of a new medical product, it is critical to ensure the integrity of the data generated by assays used to measure them.
Cliantha has developed a wide biomarker portfolio which includes over 100+ biomarkers spanning a diverse range of disease states. Cliantha works closely with clients to shape their biomarker strategy and selecting the best analytical technology by:
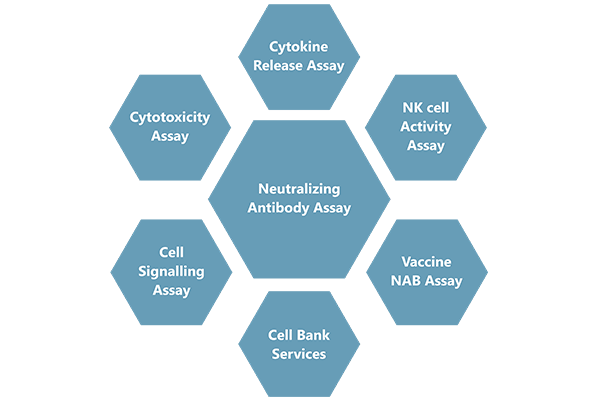
Cliantha has well equipped Biosafety level 2 (BSL-II) state-of-the-art cell culture laboratory to provide full support for large molecule bio-analysis including neutralizing antibody assay, In-vitro assays and vaccine immunogenicity.
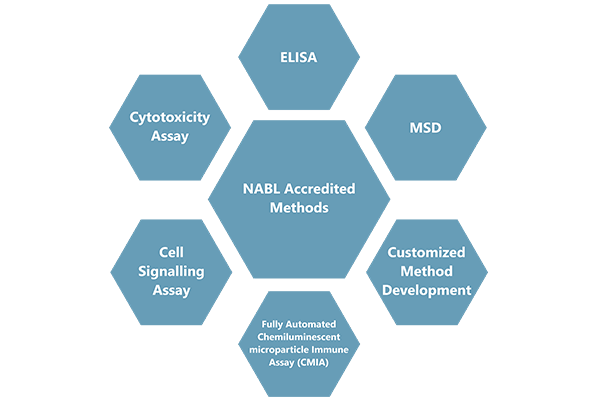
Cliantha has well equipped state of art analytical facility to provide NABL accredited vaccine immunogenicity services for regulatory submissions. Our lab has analyzed 25000+ samples from 4000+ subjects from attenuated (live) vaccines, inactivated vaccines, toxoid vaccines and conjugate vaccines
Our vaccine experience includes method validation and sample analysis for
Technology platforms for vaccine analysis,
Biomarkers are increasingly used to assess the effects of new drugs and therapeutic biological products in patient populations. Because of the important roles biomarkers can play in evaluating the safety, activity, or effectiveness of a new medical product, it is critical to ensure the integrity of the data generated by assays used to measure them.
Cliantha has developed a wide biomarker portfolio which includes over 100+ biomarkers spanning a diverse range of disease states. Cliantha works closely with clients to shape their biomarker strategy and selecting the best analytical technology by:


Cliantha has well equipped Biosafety level 2 (BSL-II) state-of-the-art cell culture laboratory to provide full support for large molecule bio-analysis including neutralizing antibody assay, In-vitro assays and vaccine immunogenicity.

Clinical development of vaccines requires a specific set of specialized assays to demonstrate the immunogenicity of the vaccine. Ideally, these assays should measure immune responses that correlate with protection against disease. Developing assays for new-generation vaccines usually requires working with cells, pathogens, antigens or assay controls that are not readily available. Validation of these assays involve many challenges as validation requirements are not yet fully specified in regulatory guidelines or White Papers. Our large molecule team has demonstrated experience in conducting numerous CT vaccine studies.
Our vaccine experience includes method validation and sample analysis for
Technology platforms for vaccine analysis,

Cliantha Research offers cost effective microbiology contract research services to companies involved in medical devices and antimicrobial products.